Written by Alejandra Feliciano
Worldwide diversity loss across taxa has accelerated exponentially in the last decades (Ceballos et al. 2015). Landscape professionals seek science-based best practices to battle this ongoing diversity crisis. But how exactly are plants involved in ongoing mass extinction?
[1] “The Age of Man” also known as the Holocene, defined as the period following the last ice age which coincides with the explosion of Homo sapiens populations and the mass extinction of numerous taxa worldwide (Ceballos et al. 2015).
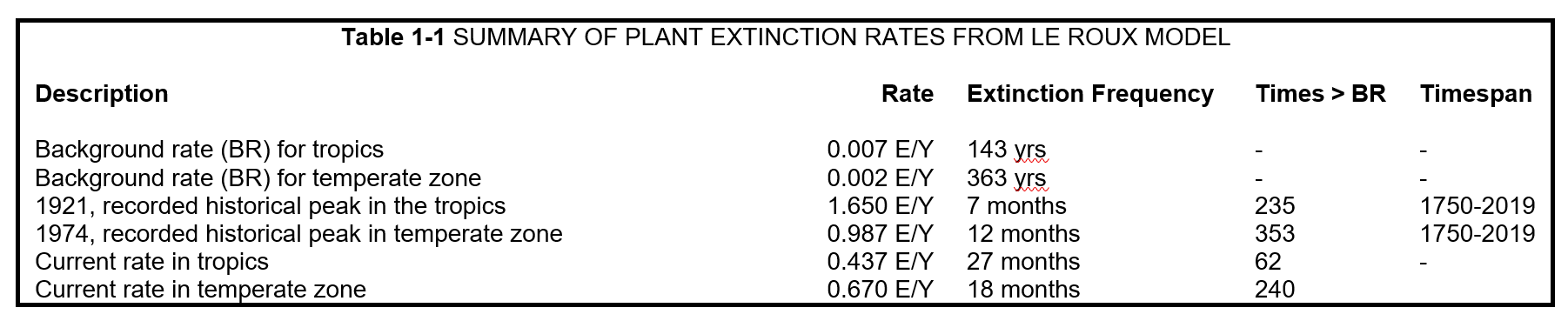
During the early part of the Anthropocene[1], plant extinctions were extremely infrequent. It is estimated that the geological background extinction rate for plants in the temperate zone was 0.00279 E/Y (Le Roux et al. 2019). This means that, on average, one plant species would go extinct every 363 years. After the industrial revolution, the rate spiked and has since been on a slow decline, maintaining anthropogenically high levels. A recent study estimates the current extinction rate in the temperate zone to be 0.67 E/Y, or one plant extinction every 18 months (Le Roux et al. 2019).
[2] The calculation here is 0.65 S/MSY = 0.65 x 10-6 S/Y = 1 species / 1,538,461 years.
[3] The calculation here is 8.40 S/MSY = 8.40 x 10-6 S/Y = 1 species / 119,047 years.
[4] Ecological succession: the process of change in the species structure of an ecological community over time (“Ecological succession” 2020).
These rates do not account for speciation[2]. The worldwide background rate of speciation is estimated to be one new plant species occurring every 1.5 to 7.1 million years[3] (Vellend et al. 2017). Interestingly, globalization has provided new opportunities for plant hybridization and speciation, although its net effect on global richness is unknown (Thomas 2015). To the author’s knowledge, no studies have been conducted to estimate contemporary speciation rates, in part due to lack of reliable data (Humphreys et al. 2019) . It has been suggested the rate could be as high as one new plant species every 119,047 years[4] (Thomas 2015).
Although the number of documented extinctions is low, as many as 40,000 species are thought to be “dead species walking” (Bridgewater et al. 2019). This extinction debt occurs when living plants are unable to reproduce and become their last generation. Unlike mammalian extinctions, which can take about 13 years to occur, plant extinctions can take anywhere between 100 to 500 years (Cronk 2016), undergoing six distinct stages (Downey and Richardson 2016). Because plant extinction is a slow process, these functionally extinct species may remain present for centuries, falling largely unaccounted for in extinction counts.
Additionally, the number of plants threatened with extinction is estimated to be high. Recent estimates place the number of endangered plant species at around 50,000 (Cronk 2016). There are 381,910 described plant species (Willis 2017), but roughly 110,000 remain undiscovered because they are rare, and likely threatened, raising the worldwide number of plant species closer to 500,000 (Corlett 2016). Still, these estimates may not be representative of the global extinction risk, since 94% of described species have not yet been assessed for conservation status and are not being tracked due to lack of funding (Corlett 2016).
Diversity loss beyond plant extinctions: evenness and range can impact richness at heterotrophic levels
[5] Richness: the number of distinct plant species within an ecological community.
[6] Evenness: the distribution of species abundance within an ecological community.
[7] Heterotroph: an organism that cannot produce its own food, instead taking nutrition from other sources (“Heterotroph” 2020).
[8] Relative abundance: the proportional abundance based on number of individuals for each species compared to the total number of individuals in the community.
Plants take a long time to go extinct, so their communities rarely face observable richness[5] depression. Their generally slow and declining rate of extinction might lead stakeholders to concentrate conservation efforts elsewhere. But it does not take centuries for plant populations to loose evenness[6] and habitat range, which can lead to extinction of heterotrophic[7] organisms.
An example of this are extinctions of organisms that rely on plant species maintaining baseline populations. Plant community evenness can be disturbed by the introduction of invasive species. About 6,075 plant species have been identified as invasive (Willis 2017). The recruitment of these plants can change the taxonomic histogram of the plant community, lowering the relative abundance[8] of native plants. When native populations become depressed, it can lead to co-extinctions of heterotrophs in facilitative relationships with plants, such as plant-pollinator networks (Veron et al. 2018). It is important to note although this can become an indirect pathway to plant extinction (Strona and Bradshaw 2018); there is little evidence of invasive plants causing plant extinctions through direct competition (Corlett 2016). In fact, plant invasions have been shown to spike plant speciation and may be buffering diversity loss during the Anthropocene (Thomas 2015). Net effects of invasive species on global plant diversity loss remain unknown (Thomas 2013).
The effects of range reduction are just as complex. Habitat loss from worldwide reductions in the geographical ranges of endemic plant communities is an ubiquitous culprit behind most animal extinctions (Ceballos et al. 2015). Notably, range reduction is listed as one of the main drivers of diversity loss in the tropics, where forests are loosing range at a rate of 5PgC per year (Liu et al. 2015). In temperate zones there is an opportunity for range expansion from the melting permafrost, where boreal and mixed forests are actually increasing in range by 2.3 PgC each year (Liu et al. 2015). For temperate zones, the main drivers behind range reductions are hydrological changes, habitat degradation, and the displacement of natural areas by agriculture (Le Roux et al. 2019).
Proposed Strategies
The most impactful action that can be taken to cauterize diversity loss is to preserve existing natural areas. A study in Hungary showed preservation of natural areas was the most effective among all strategies to stave off habitat loss (Biró et al. 2018). Along with preservation, diversity loss must be alleviated through strategic, targeted landscaping in urban areas.
Urban plant communities are necessary to bridge fragmented habitats
Once natural habitats have been converted to urbanized land, they are unlikely to revert back to their natural state. Because such displacement is irreversible, part of the solution must consider how to transform landscapes on these sites into part of the solution for conservation. Not all green spaces in urban areas are created equal. For example, urban parks surrounded by gray sites are ecologically isolated, and would face the same challenges of habitat fragmentation as fragmented natural areas, compounded by the instability of species turnover typical of increased edge effects (Eldegard et al. 2015).
To have the most impact, landscaped urban areas should be placed strategically to create a network of semi-natural corridors connecting previously isolated natural areas (Damschen et al. 2019). Plant species selection within these corridors should be concentrated in a successional gradient to pump species credit and alleviate extinction debt (Hanski 2000).
Green roofs can be incorporated into this strategy, specifically targeting heavily paved gray sites, which can help the connectivity of bird and invertebrate populations (Fernandez-Canero and Gonzalez-Redondo 2010, Braaker et al. 2014). Streetscapes have potential for connectivity, which have shown to support bird and small mammal populations (Bolger et al. 2001).
Similarly, agricultural sites can engage in regional campaigns to incorporate diversity corridors within under-utilized portions of the land, such as field margins, stream banks, and ditches (Thiele et al. 2018).
2. Edge effect considerations
It has been well documented that plant communities are less stable at their boundary edges than at their interior (Eldegard et al. 2015) and that urban plantings need sufficient space to have rich diversity (Beninde et al. 2015). This is known as the edge effect, where plant communities at the edge are more diverse but transitional, and less diverse but more stable in their interior (Villard and Metzger 2014). The smaller the planting area, the smaller the species that should be planted, to minimize edge effects. For example, small planting strips along local roads should be planted with a relatively short understory community to minimize edge effects. Larger planting sites, such as highway medians and green roofs, can afford larger plant material due to their larger interior space.
3. Urban plant communities can serve as conservation sites for species that have been lost or will become lost to the wild in the next decades
The availability and quality of sources for rare plant reintroduction projects are an important aspect in the conservation of highly threatened species (Maschinski et al. 2012). Urban landscapes can play an important role in both ex-situ and in-situ rare plant reintroductions (Ren et al. 2014). Urban landscapes can serve as a repository to introduce species back to their native ranges, much like botanical garden collections. Regardless of wild reintroduction success, conservation is still possible within planted urban environments. Some rare plants may establish more successfully in early-successional[4] urban landscapes than within in their historical geographical ranges due to habitat degradation.
4. Dominance design could help build resilience against exotic invasion and help shore diversity conservation
The community assembly filters that control colonization dynamics, abiotic compatibility, and interactions among plants, affect the long-term diversity of planted landscapes (Chang and HilleRisLambers 2016). Although these functional interactions have been understood theoretically, only recently quantitative methods have been developed that offer the potential of creating forecasting models of complex community assembly outcomes (Pierce et al. 2017). The ability to test potential planted community combinations against the surrounding weed pool for dominance could arm landscape designers and conservation ecologists with the information necessary to design ecologically successful landscapes that naturally outcompete weeds, are functionally resilient, require less maintenance, and ultimately support diversity conservation goals.
Works Cited
Beninde, J., M. Veith, and A. Hochkirch. 2015. Biodiversity in cities needs space: a meta-analysis of factors determining intra-urban biodiversity variation. Ecology Letters 18:581–592.
Biró, M., J. Bölöni, and Z. Molnár. 2018. Use of long-term data to evaluate loss and endangerment status of Natura 2000 habitats and effects of protected areas. Conservation Biology 32:660–671.
Bolger, D. T., T. A. Scott, and J. T. Rotenberry. 2001. Use of corridor-like landscape structures by bird and small mammal species. Biological Conservation 102:213–224.
Braaker, S., J. Ghazoul, M. K. Obrist, and M. Moretti. 2014. Habitat connectivity shapes urban arthropod communities: the key role of green roofs. Ecology 95:1010–1021.
Bridgewater, P., A. Loyau, and D. S. Schmeller. 2019. The seventh plenary of the intergovernmental platform for biodiversity and ecosystem services (IPBES-7): a global assessment and a reshaping of IPBES. Biodiversity and Conservation 28:2457–2461.
Ceballos, G., P. R. Ehrlich, A. D. Barnosky, A. García, R. M. Pringle, and T. M. Palmer. 2015. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Science Advances 1:e1400253.
Chang, C., and J. HilleRisLambers. 2016. Integrating succession and community assembly perspectives. F1000Research 5.
Corlett, R. T. 2016. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Diversity 38:10–16.
Cronk, Q. 2016. Plant extinctions take time. Science 353:446–447.
Damschen, E. I., L. A. Brudvig, M. A. Burt, R. J. Fletcher, N. M. Haddad, D. J. Levey, J. L. Orrock, J. Resasco, and J. J. Tewksbury. 2019. Ongoing accumulation of plant diversity through habitat connectivity in an 18-year experiment. Science 365:1478–1480.
Downey, P. O., and D. M. Richardson. 2016. Alien plant invasions and native plant extinctions: a six-threshold framework. AoB PLANTS 8.
Ecological succession. 2020, June 14. .
Eldegard, K., Ø. Totland, and S. R. Moe. 2015. Edge effects on plant communities along power line clearings. Journal of Applied Ecology 52:871–880.
Fernandez-Canero, R., and P. Gonzalez-Redondo. 2010. Green Roofs as a Habitat for Birds: A Review. Journal Of Animal And Veterinary Advances 9:2041–2052.
Hanski, I. 2000. Extinction debt and species credit in boreal forests: modelling the consequences of different approaches to biodiversity conservation. Annales Zoologici Fennici 37:271–280.
Heterotroph. 2020, August 4. .
Humphreys, A. M., R. Govaerts, S. Z. Ficinski, E. N. Lughadha, and M. S. Vorontsova. 2019. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nature Ecology & Evolution 3:1043–1047.
Le Roux, J. J., C. Hui, M. L. Castillo, J. M. Iriondo, J.-H. Keet, A. A. Khapugin, F. Médail, M. Rejmánek, G. Theron, F. A. Yannelli, and H. Hirsch. 2019. Recent Anthropogenic Plant Extinctions Differ in Biodiversity Hotspots and Coldspots. Current Biology 29:2912-2918.e2.
Liu, Y. Y., A. I. J. M. van Dijk, R. A. M. de Jeu, J. G. Canadell, M. F. McCabe, J. P. Evans, and G. Wang. 2015. Recent reversal in loss of global terrestrial biomass. Nature Climate Change 5:470–474.
Maschinski, J., K. E. Haskins, M. Center for Plant Conservation Saint Louis, and Society for Ecological Restoration. 2012. Plant Reintroduction in a Changing Climate Promises and Perils. 1st ed. 2012. Island Press/Center for Resource Economics : Imprint: Island Press, Washington, DC.
Pierce, S., D. Negreiros, B. E. L. Cerabolini, J. Kattge, S. Díaz, M. Kleyer, B. Shipley, S. J. Wright, N. A. Soudzilovskaia, V. G. Onipchenko, P. M. van Bodegom, C. Frenette-Dussault, E. Weiher, B. X. Pinho, J. H. C. Cornelissen, J. P. Grime, K. Thompson, R. Hunt, P. J. Wilson, G. Buffa, O. C. Nyakunga, P. B. Reich, M. Caccianiga, F. Mangili, R. M. Ceriani, A. Luzzaro, G. Brusa, A. Siefert, N. P. U. Barbosa, F. S. Chapin III, W. K. Cornwell, J. Fang, G. W. Fernandes, E. Garnier, S. Le Stradic, J. Peñuelas, F. P. L. Melo, A. Slaviero, M. Tabarelli, and D. Tampucci. 2017. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Functional Ecology 31:444–457.
Ren, H., S. Jian, H. Liu, Q. Zhang, and H. Lu. 2014. Advances in the reintroduction of rare and endangered wild plant species. Science China Life Sciences 57:603–609.
Speciation. (n.d.). . https://www.lexico.com/en/definition/speciation.
Strona, G., and C. J. A. Bradshaw. 2018. Co-extinctions annihilate planetary life during extreme environmental change. Scientific reports 8:16724.
Thiele, J., S. Kellner, S. Buchholz, and J. Schirmel. 2018. Connectivity or area: what drives plant species richness in habitat corridors? Landscape Ecology 33:173–181.
Thomas, C. D. 2013. The Anthropocene could raise biological diversity. Nature 502:7–7.
Thomas, C. D. 2015. Rapid acceleration of plant speciation during the Anthropocene. Trends in Ecology & Evolution 30:448–455.
Vellend, M., L. Baeten, A. Becker-Scarpitta, V. Boucher-Lalonde, J. L. McCune, J. Messier, I. H. Myers-Smith, and D. F. Sax. 2017. Plant Biodiversity Change Across Scales During the Anthropocene. Annual Review of Plant Biology 68:563–586.
Veron, S., C. Fontaine, N. Dubos, P. Clergeau, and S. Pavoine. 2018. Predicting the impacts of co-extinctions on phylogenetic diversity in mutualistic networks. Biological Conservation 219:161–171.
Villard, M.-A., and J. P. Metzger. 2014. Beyond the fragmentation debate: a conceptual model to predict when habitat configuration really matters. Journal of Applied Ecology 51:309–318.
Willis, K. J. 2017. State of the world’s plants report - 2017. Royal Botanic Gardens.